Laboratory Water Purification System Difference, Types, Uses Comparison
Water purification systems remove a wide variety of contaminants from feed water (tap water or pre-treated water) to produce pure water ideal for a host of applications within the lab.
Common Laboratory Tap Water Contaminants
Common contaminants within tap water include enzymes (nucleases and proteases), gases, organics, microbes (bacteria, viruses), colloids and inorganic ions. Water purity systems are designed to filter specific classes of contaminants endemic to certain applications; other primary differences between systems include tank capacity, production rate, grade of water produced, scalability, level of maintenance, and remote dispensing.
What Is Lab-Grade Water?
As lab-grade water is used in a broad range of processes, ridding the feed water of contamination sources is critical to ensuring accurate results.
What Are the Most Common Contaminants in Laboratory Water?
The main categories of common water-borne contaminants are microbes, enzymes, inorganic charged ions, colloids, gases and organics.
Water Contamination Sources - Cell Culture, PCR Reactions, and Lab Research
Microbes, such as bacteria and viruses, will influence the results of cell culture studies, PCR reactions, and environmental research. Enzymes, such as nucleases and proteases, will break-down nucleic acid and protein targets, subverting the results of microarrays, DNA sequencing and high-throughput screening (HTS) experiments.
Charged inorganic ions, such as magnesium, calcium, and sodium, act as catalysts in chemical reactions, affecting immunoassays, like ELISAs. Colloids, sediment and suspended organic matter damage analytical instruments, such as high-performance liquid chromatography (HPLC) pumps and injectors, gas chromatography (GC) systems, and mass spectrometers.
Dissolved gases, such as carbon dioxide, nitrogen, oxygen and ammonia, alter the water PH by forming acids and bases during ionization. Gases commonly form bubbles in water, skewing the results of liquid particle counting or spectrophotometric measurements.
Common organics found in feed water, including detergents, oils and solvents, interfere with experiments by increasing background noise and promoting microbial growth.
How Do Water Purification Systems Remove Contaminants from Lab Water?
Water purity systems in laboratories utilize a number of different technologies to remove contaminants from feed water; each technology has different benefits and limitations. For critical applications requiring ultra-pure water, labs may develop multi-stage purification systems combining several filtration or adsorption technologies.
Methods of Laboratory Water Purification
Distillation, the simplest form of water purification, involves boiling water in a flask connected to a cooling coil. The cooling coil condenses the water vapor back into liquid form and the water is eluted into a separate beaker or storage tank. Distillation removes a wide variety of contaminants, but the process is time-consuming and laborious. In addition, certain contaminants, such as organics, with boiling points over 100°C, will be boiled alongside the water and condensed into the final elution.
Ion exchange is a gravimetric process whereby water percolates down a vertical column filled with ion exchange, bead-based resins. The round, semi-porous beads exchange ions with the feed water, such as hydrogen ions for cations, like sodium ions. This low-cost, low-capacity purification method effectively removes inorganic charged ions from feed water, but does not remove organics or microbes. Ion exchange is commonly used as a pretreatment step before further water filtration.
Activated charcoal is a filtration process designed to remove organics molecules from feed water. As water passes through the carbon filter, organic molecules bind to the pore walls through van der Waals forces. Charcoal filters last longer than other filtration media, but carbon filters do not remove inorganic ions, particulates or colloids from water.
Ultrafiltration is a purification technology involving water passing through a semipermeable membrane. Suspended and high molecular weight solids are retained by the membrane filter, while low molecular weight solutes pass through the membrane into the permeate. Ultrafiltration effectively removes most microorganisms and particles from feed water, but organic molecules and inorganic ions are not filtered away.
Reverse osmosis is a purification technology that uses an applied force to overcome osmotic pressure caused by chemical potential differences in the solvent. During reverse osmosis (RO), the solvent passes through the semipermeable membrane while the solute is retained on the pressurized side. Although reverse osmosis removes all types of contaminants, the flow rate remains low; causing most labs to run RO systems overnight to ensure sufficient water is available each workday.
Ultraviolet (UV) germicidal radiation employs short-wavelength UV light to inactivate microorganisms. Beyond destroying a wide variety of microbes, UV light (emitted at 254 nm) oxidizes organic compounds to produce water with TOC levels below 5 ppb (parts per billion). However, UV light does not remove colloids, particles or inorganic ions from water.
Lab Water Purification Uses and Applications
Laboratory water purification systems are commonly used in many applications, including environmental science, pharmaceutical research and development, clinical diagnostics, medical microbiology, life science, and academic research.
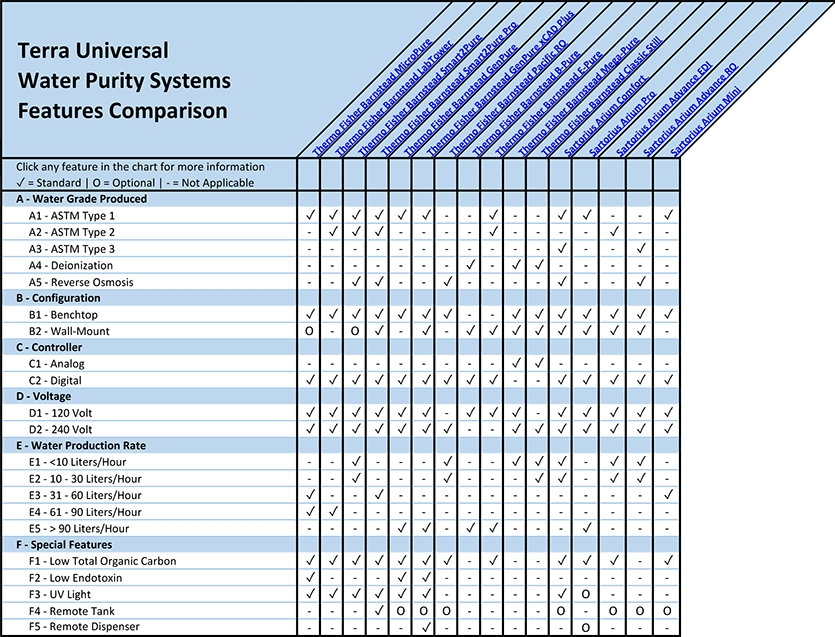
A - Water Grade Produced
(back to chart)
A1 - ASTM Type 1 Water
ASTM type 1 water, the most purified water grade, is generally reserved for critical applications, such as HPLC, GC and ICP-MS, or analytical techniques such as real-time PCR, DNA sequencing, and genetic screening. As type 1 water is more expensive to produce than lower grades, using type 1 water for non-critical applications is cost prohibitive.
A2 - ASTM Type 2 Water
ASTM type 2 water systems are used to make PH-neutral buffer, cell and tissue culture media, and common bulk reagents. Type 2 water is commonly used as a pre-treated feed for purification systems producing type 1 water.
A3 - ASTM Type 3 Water
ASTM type 3 water is used exclusively for non-critical lab work, like glassware rinsing, and feed water for reagent baths, autoclaves, test chambers, water-jacketed incubators, plant growth chambers, and disinfection systems. Type 3 water may be used as feed water for purification systems producing type 1 or type 2 water.
A4 - Deionization Water Systems
Ion exchange is a gravimetric process whereby water percolates down a vertical column filled with ion exchange, bead-based resins. The round, semi-porous beads exchange ions with the feed water, such as hydrogen ions for cations, like sodium ions. This low-cost, low-capacity purification method effectively removes inorganic charged ions from feed water, but does not remove organics or microbes. Ion exchange is commonly used as a pretreatment step before further water filtration.
Currently available DI water systems include Thermo Fisher’s B-Pure and Mega-Pure lines.
A5 - Reverse Osmosis Water Systems
Reverse osmosis is a purification technology that uses an applied force to overcome osmotic pressure caused by chemical potential differences in the solvent. During reverse osmosis (RO), the solvent passes through the semipermeable membrane while the solute is retained on the pressurized side. Although reverse osmosis removes all types of contaminants, the flow rate remains low; causing most labs to run RO systems overnight to ensure sufficient water is available each workday.
Currently available RO water purification systems include Thermo Fisher’s Pacific RO and Sartorius’ Arium Advance RO lines.
B - Water Purification System Configuration
(back to chart)
B1 - Benchtop Water Purification Systems
Benchtop water purification systems, like Thermo Fisher’s MicroPure and Sartorius’ Arium Comfort, are designed for point-of-use applications and overnight, bulk water production. Although benchtop systems consume more workspace than wall-mounted systems, they include larger tanks and more available accessories (such as UV lamps and ultra-filters) than their wall-mounted counterparts.
B2 - Wall-Mounted Water Purification Systems
Wall-mounted purification systems, like Thermo Fisher’s GenPure xCAD Plus and Sartorius’ Arium Smart Station, include a mounting bracket and up to 3 optional remote dispensers. Although wall-mounted systems include smaller tanks than benchtop units, they free up precious work space for other critical equipment.
C - Water Purification System Controllers
(back to chart)
C1 - Analog Water Purification Systems
Economical water purification systems, like Thermo Fisher’s MegaPure and Classic Still lines, include analog controllers featuring feed water solenoid valves for automatic operation. Digital controllers include additional features, such as reservoir-level indicators, user-programmable methods, filter replacement alarms, data export, and GLP-compliant documentation.
C2 - Digital Water Purification Systems
Water purification systems, like Thermo Fisher’s Smart2Pure Pro and LabTower, include digital controllers with integrated feed water monitoring, GLP-compliant documentation, data export routines, operating mode status indicators, user-programmable protocols, reservoir-level indicators and cartridge change alarms.
D - Water Purification System Voltage
(back to chart)
120-volt connections are suitable for standard laboratory power outlets in the United States. 240-volt connections, common in Mainland Europe, require less current (amperage) and smaller conductors than equipment designed to operate at 120-volt.
E - Water Production Rate
Combined with the grade of water produced, the flow rate is the most crucial attribute of each water purification system. To calculate the total system capacity, the flow rate (listed in liters per hour or gallons per hour) must be paired with the reservoir capacity (listed in liters or gallons).
Smaller research labs use lower-volume, point-of-use systems, such as Thermo Fisher’s Pacific RO or Sartorius’ Arium Advance EDI.
High-throughput labs require high-volume systems exceeding 90 liters per hour, like Thermo Fisher’s GenPure or B-Pure lines, with storage tanks capable of holding up to 200 liters of purified water. High-throughput labs commonly run their water purification systems overnight to ensure the reservoir is filled to capacity at the outset of each workday, preventing downtime associated with a shortage of lab-grade water.
F - Special Features
(back to chart)
F1 - Low Total Organic Carbon (TOC)
TOC is the measure of the total amount of carbon in organic compounds within purified water. Although no direct correlation exists between total organic carbon and the total concentration of organic compounds, TOC is used as a general indicator of the estimated level of organic contamination in pure water.
Common organics found in feed water, including detergents, oils and solvents, interfere with experiments by increasing background noise and promoting microbial growth. TOC levels in river water generally rest around 7 ppm (parts per million), whereas TOC levels in seawater (700 ppb (parts per billion)) and drinking water (100 ppb) are significantly lower. Ultra-pure, lab-grade water must contain fewer than 10 ppb of total organic carbon to meet ASTM standards. Thermo Fisher’s E-Pure and GenPure systems produce purified water with TOC levels at, or below, 5 ppb (parts per billion).
F2 - Low Endotoxin
Endotoxins are a class of lipopolysaccharides (LPS) found in the outer membrane of most Gram-negative bacteria. Endotoxins present in purified water impact the growth and cloning function of cell lines lacking endotoxin receptors. In addition to their negative impact on cell culture research, endotoxins also act as a general indicator of microbial levels within purified water. Ultrafiltration and ultraviolet (UV) light exposure are effective methods to reduce endotoxin levels in feed water.
Thermo Fisher’s MicroPure system produces purified water virtually free of endotoxins (<0.001 EU/ml).
F3 - UV Light
Ultraviolet (UV) germicidal radiation employs short-wavelength UV light to inactivate microorganisms. Beyond destroying a wide variety of microbes, UV light (emitted at 254 nm) oxidizes organic compounds to produce water with TOC levels below 5 ppb (parts per billion). However, UV light does not remove colloids, particles or inorganic ions from water.
Thermo Fisher’s Smart2Pure and LabTower water purification systems include optional UV upgrades.
F4 - Remote Tank
For high-throughput labs, water purification systems with remote tanks provide the option to run water purification systems overnight to ensure the reservoir is filled to capacity at the outset of each workday, preventing downtime associated with a shortage of lab-grade water.
Sartorius’ Arium Comfort and Arium Advance EDI (Type 2 | Type 3) systems include optional remote tanks.
F5: Remote Dispenser
Thermo Fisher’s Barnstead GenPure xCAD Plus ultrapure water purification systems include optional remote dispensers for point-of-use applications. The central purity system is compatible with up to 3 remote dispensers placed throughout the lab.
Where Can I Buy Water Purification Systems Online?
Laboratory-Equipment.com is a specialty division of Terra Universal. For nearly 40 years, Terra Universal has served the life science, pharmaceutical, biotechnology, and medical device markets. Customers appreciate a worldwide network of reps, factory-direct support, and ready-to-ship items available from Terra's on-shore manufacturing and warehouse facilities in Fullerton, California.
Shop water purification systems online to compare a variety of pharmaceutical, laboratory, life science research, and analytical environments.
Contact a Laboratory-equipment.com specialist through web chat, email, or phone for pricing or a same-day quote.
Laboratory-Equipment.com | U.S. Customer Service
Email: Info@Laboratory-Equipment.com
Phone: (714) 578-6016



